
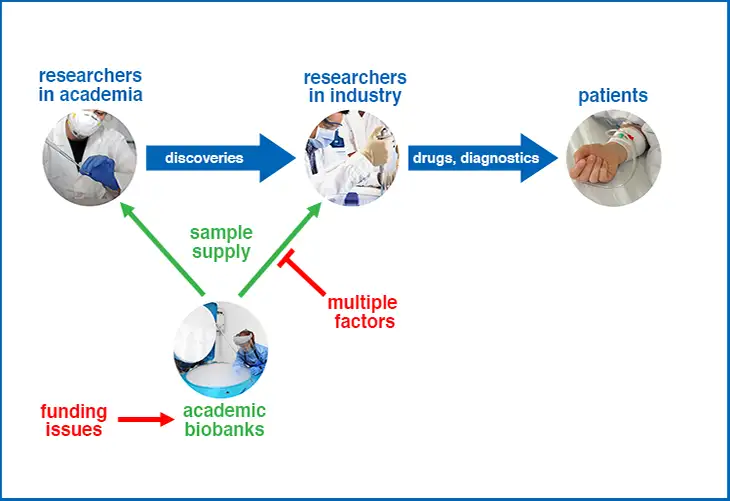
Academic biobanks have a dual role: they must support two actors in a production that advances in patient care. The two actors they need to support are (1) researchers in academia and (2) researchers in industry. It is only by the efforts of both these actors that discoveries in academia can be translated into drug and diagnostic products for patient care.
Academic Biobanks Must Provide Human Biospecimen Access To Both Academia And Industry
Academic biobanks have a moral responsibility to provide biospecimens to researchers in industry. Academic biobanks receive public funding, and industry needs their support for the public good. Without reliable biospecimens, industry can not develop new and improved therapies and diagnostics.
Cooperation with industry is one way academic biobanks can achieve financial sustainability. The biobank can charge a fee for service. They can also get industry funding for collaborative research. There is no question that biobanks need this financial support. Experience over the past 20 years has shown us this.
Furthermore, pharma-academia collaboration can be very beneficial for other reasons. It may allow the transfer of skills and expertise between academia and industry. In addition, it may allow sharing of expensive facilities and equipment.
Multiple Factors Inhibit Human Tissue Provision To Industry
There are ‘multiple factors’ that inhibit academic biobanks from providing biosamples to industry. As a result, there are negative consequences:
- Industry researchers lack biospecimens which makes their research more difficult.
- Academic biobanks lack industry funding which reduces their ability to serve both academia and industry.
So what are these ‘multiple factors’?
Some are practical issues like insufficient funding. They include cultural issues like the different mindsets of industry and academia. Also, they include ethical issues, like concerns about the commercialisation of biobanking. Sometimes, it must be said, are sensitive issues that are not easy to discuss.
However, what is at stake here is the welfare of future patients. New and improved therapeutics and diagnostics depend on research in both industry and academia. Unfortunately, biospecimen access problems stand in the way of this research. So the ‘multiple factors’ need examination in broad daylight. They need open discussion.
So we discuss them here in this series of blog posts. Also, in the following article: Why Hospital Biobanks Need To Supply Industry – and Why There Is a Blockage in the System, August 9 2021
This article is on the Biosample Hub blog. Other posts in this blog include:
- Why Academic / Hospital Biobanks Should Provide Biosamples to Industry
- Ensuring Public Support For Biobank Cooperation With Industry
- The Main Actors Providing Biosamples To Industry
- Biosample Needs of Different Industry Players
- The Dual Role of Academic Biobanks
- What is Commodification?
- Acceptable Transactions in Biobanking
- Why Biobank Access Policies Should Be Publicly Available
- Biospecimen Provenance: What Researchers Need To Know
The Biosample Hub platform https://biosamplehub.org/ encourages ethical sourcing of biosamples for the industry sector.
