Public Attitudes to Biobank Cooperation With Industry
In general, industry involvement in research tends to lower public trust. We see this effect whether the industry sector funds or conducts the investigation. Here are a few critical studies on the subject.
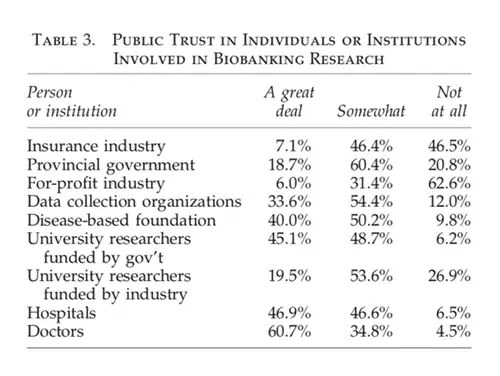
A Canadian Study
Caulfield et al. described the results of a 2012 telephone survey of public members in Alberta, Canada, in the following 2012 paper. Biobanking, Consent, and Control: A Survey of Albertans on Key Research Ethics Issues. This examined level of trust in various users and custodians of health information.
Results show that the insurance and for-profit industries were the least trusted. In contrast, doctors and hospitals were the most trusted. However, it is essential to realise these results are not all or nothing. For example, even doctors who are the most trusted group are only somewhat trusted by 34.8% and not at all trusted by 4.5%.
A Review of US Studies
Garrison et al. reviewed 48 studies on attitudes to biobank research. Also, on attitudes to broad consent and data sharing. They reported this in the following 2016 article—a systematic literature review of individuals’ perspectives on broad consent and data sharing in the United States. In summary, most participants were willing to share data with pharmaceutical company researchers. Yet, a smaller percentage were willing to share with industry than academic researchers.
A Mixed Methods UK Study
Lewis et al. conducted a quantitative survey combined with focus group discussions. They published this UK study in a 2013 article. Public views on the donation and use of human biological samples in biomedical research: a mixed-methods study. The findings of the survey were as follows. Most responders were willing to donate biosamples to National Health Service hospitals (84%). Fewer for medical research charities (79%) and universities (68%). Fewer still for diagnostic companies (63%) and pharmaceutical companies (56%).
Based on the focus group discussions, they report as follows. There was some initial negativity towards pharmaceutical companies conducting research. This negativity was because of their commercial, profit-making nature. Also, due to concerns that they ‘exploit patients’. Yet, other members of the group often addressed such concerns. They acknowledged that the commercialisation of research was ‘a fact of life’. Also, that pharmaceutical companies need to make money to keep their research going.
A Focus Group Study
A 2017 public dialogue study in the UK had the following title. Consent to use human tissue and linked health data in health research. This study involved 75 selected volunteers in focus group discussions about consent. The participants’ most common red lines were as follows. First, they felt there should be no access for commercial companies like insurance or marketing companies. This response was because they would be using data to sell a product. Second, some did not want pharma companies to have access to their data. Yet, after explaining the role of pharma in research, almost all were less concerned. Finally, the study found that few participants had heard of biobanks before.

The Need For Public Education About Biobank Research
As mentioned, few participants in the 2017 UK study had heard of biobanks before. This was despite the outstanding success of the UK Biobank, a project involving 1% of the UK population. This lack of awareness is not unexpected. The Eurobarometer 73.1 survey reported a similar finding. The survey found that many European citizens are unaware of biobanks. Indeed, two-thirds of respondents had not heard about biobanks before their interview (Europeans and Biotechnology in 2010: Winds of Change? – A report to the European Commission’s Directorate-General for Research
July 2013).
July 2013).
Bearing in mind this lack of public awareness, here are a couple of observations.
-
Simple questionnaires about attitudes to biobanking may not give meaningful results. Some preliminary education and discussion are necessary. So focus group studies, where participants can learn from discussion seem preferable.
-
Public/patient support for biobank cooperation with industry requires education. One obvious place for this education is during the informed consent process. At this stage, consent staff can explain why the industry sector needs biospecimens. They can also explain the benefits. That is to say, new therapeutics and diagnostics may result.
The Need For Biobank Transparency About Biosamples Access
We live in “The Age of Transparency”. We find information on the internet with great ease. So these days, it is more challenging than ever to accept when we can’t find information. We also have a growing expectation that institutions and companies should be transparent. Here is a lovely quote to illustrate the importance of transparency!
‘A lack of transparency results in distrust and a deep sense of insecurity’. Dalai Lama
Biobanks must be more transparent to ensure public support for their cooperation with the industry sector. This applies especially to how they distribute biospecimens. This need for openness starts with the informed consent process.
The revised US Common Rule (2017) requires that informed consent includes mention of commercial profits. Thus, informed consent must state whether samples could be used ‘for commercial profit.’ Also, it must say whether contributors will share in such profit themselves. Federal Policy for the Protection of Human Subjects. Final rule. Fed Regist. 2017;82(12):7149–274.
The relevant UK MRC guidelines state the following. ‘Where possible, participants should know when their sample or products derived from it may be used by the commercial sector, and the potential benefits of this access. It is also important to let the participant know they will not be entitled to a share of any profits that might ensue… ‘Human Tissue and Biological Samples for Use in Research (2014).’
Another way biobanks can be transparent is by giving annual reports on distributing samples.
Conclusions
The public is less trusting of industry research on biospecimens, as compared with university research. Yet, when educated about the benefits of industry research, they are more supportive. In Europe, there is a lack of public awareness about biobanks. Therefore, there is a need for public education about biobanks and why they need to cooperate with industry. In addition, biobanks need to be transparent about how they distribute samples. These measures will help to maintain public trust. This trust will help to ensure public support for biobank cooperation with industry.
This article is on the Biosample Hub blog. Other posts in this blog include:
- Why Academic / Hospital Biobanks Should Provide Biosamples to Industry
- Ensuring Public Support For Biobank Cooperation With Industry
- The Main Actors Providing Biosamples To Industry
- Biosample Needs of Different Industry Players
- The Dual Role of Academic Biobanks
- What is Commodification?
- Acceptable Transactions in Biobanking
- Why Biobank Access Policies Should Be Publicly Available
- Biospecimen Provenance: What Researchers Need To Know
The Biosample Hub platform https://biosamplehub.org/ encourages ethical sourcing of biosamples for the industry sector.