Public Attitudes Toward Biospecimen Access and Industry Cooperation
Biospecimen access plays a crucial role in the development of vaccines, diagnostics, and therapeutics. However, public trust and support for industry cooperation in this area are often debated. In general, industry involvement in research tends to reduce public trust, whether the industry funds or directly conducts the investigation. Below, we explore several studies on public perceptions of biospecimen access and cooperation between biobanks and industry.
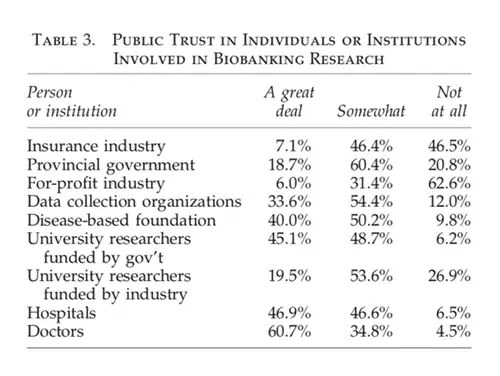
A Canadian Study on Biospecimen Access
Caulfield et al. conducted a 2012 telephone survey in Alberta, Canada, focusing on public trust in various users and custodians of health information, including those involved in biospecimen access.
The study, Biobanking, Consent, and Control: A Survey of Albertans on Key Research Ethics Issues (Biopreservation and Biobanking 2012), found that the insurance and for-profit sectors were the least trusted entities for managing biospecimens, while doctors and hospitals were the most trusted. Notably, even the most trusted group—doctors—was only “somewhat” trusted by 34.8% of respondents.
A Review of U.S. Studies on Human Biospecimen Access
Garrison et al. reviewed 48 studies on attitudes toward biobank research, focusing on broad consent and data sharing in human biospecimen access. Their 2016 article, A Systematic Literature Review of Individuals’ Perspectives on Broad Consent and Data Sharing in the United States, found that while most participants were willing to share their biospecimens with pharmaceutical companies, fewer were comfortable sharing with industry compared to academic researchers.
A U.K. Study: Biospecimen Access Platforms and Public Perception
Lewis et al. explored public views on biospecimen donation for biomedical research through a mixed-methods study, combining quantitative surveys with focus group discussions. Their 2013 article, Public Views on the Donation and Use of Human Biological Samples in Biomedical Research: A Mixed-Methods Study, revealed that 84% of respondents were willing to donate biospecimens to National Health Service (NHS) hospitals. This willingness decreased for other institutions, such as medical research charities (79%), universities (68%), diagnostic companies (63%), and pharmaceutical companies (56%).
In focus groups, participants expressed initial negativity toward pharmaceutical companies due to their profit-driven nature. However, discussions within the groups often helped participants understand the necessity of commercial research to maintain a sustainable biospecimen access platform. Some participants even acknowledged that commercial involvement in biobank access platforms is essential to keep research moving forward.
A U.K. Focus Group Study on Human Biospecimen Access
In 2017, a U.K. study titled Consent to Use Human Tissue and Linked Health Data in Health Research involved 75 participants in focus group discussions about consent and human biospecimen access. The study found that participants were particularly concerned about insurance and marketing companies accessing their biospecimens. However, after receiving information about the role of pharmaceutical companies in developing new treatments, most participants became less opposed to sharing their biospecimens with the industry.

Public Education: A Key to Biospecimen Access
The 2017 U.K. study highlighted a general lack of public awareness about biobanks, even among those familiar with the highly successful U.K. Biobank, which involves around 1% of the U.K. population. This finding aligns with the Eurobarometer 73.1 survey, which reported that two-thirds of European respondents had never heard of biobanks (Europeans and Biotechnology in 2010: Winds of Change?).
Given this lack of awareness, public education is essential for fostering support for human biospecimen access, particularly through industry collaborations. Simple surveys on public attitudes may not capture informed opinions without some preliminary education. Focus groups that allow participants to engage in discussion are more effective in this regard.
One effective platform for education is the informed consent process, during which participants can learn about the importance of biospecimen access for industry. Consent staff should explain how biospecimen access platforms contribute to the development of new vaccines, therapeutics, and diagnostics, emphasizing the critical role that industry plays in advancing these innovations.
Transparency and Biospecimen Access Platforms
In today’s “Age of Transparency,” public trust is heavily tied to how open institutions are about their processes, including biospecimen access. People expect transparency from both institutions and companies, especially when it comes to how biospecimens are distributed. This is particularly relevant for biobank access platforms, which manage biospecimen access for research.
To maintain public trust, biobanks must be transparent about how they distribute biospecimens, especially to industry partners. This transparency should begin during the informed consent process, where participants must be made aware if their samples might be used for commercial profit and whether they will share in any profits.
In the U.S., the revised Common Rule (2017) mandates that informed consent documents disclose potential commercial profits from biospecimen use. Similarly, the U.K.’s Medical Research Council (MRC) guidelines state that participants should be informed if their samples might be accessed by commercial entities and should be made aware of the benefits of this biospecimen access. Importantly, participants should also be informed that they will not be entitled to any profits resulting from the use of their biospecimens.
Ensuring Industry Access to Biospecimens
Public trust in industry research on biospecimens is generally lower compared to university-led research. However, as studies show, once people understand the benefits of industry cooperation in biospecimen research, their support increases. To ensure industry access to biospecimens through biobank access platforms, public education and transparency are vital.
In Europe, public awareness of biobanks and their cooperation with industry remains low. Addressing this gap through education on the importance of biospecimen access for the development of new medical treatments is essential. Moreover, biobanks must clearly communicate how they distribute biospecimens and provide access to industry researchers.
By fostering transparency and increasing public understanding of the role of biospecimen access platforms, biobanks can build and maintain the trust needed to support industry research. This, in turn, will ensure the continued success of biospecimen access, benefiting both the public and the advancement of medical science.
References
Garrison NA, Sathe NA, Antommaria AH, Holm IA, Sanderson SC, Smith ME, McPheeters ML, Clayton EW. A systematic literature review of individuals’ perspectives on broad consent and data sharing in the United States. Genet Med. 2016 Jul;18(7):663-71. doi: 10.1038/gim.2015.138. Epub 2015 Nov 19. PMID: 26583683; PMCID: PMC4873460.
Lewis C, Clotworthy M, Hilton S, Magee C, Robertson MJ, Stubbins LJ, Corfield J. Public views on the donation and use of human biological samples in biomedical research: a mixed methods study. BMJ Open. 2013 Aug 7;3(8):e003056. doi: 10.1136/bmjopen-2013-003056. PMID: 23929915; PMCID: PMC3740256.
Other posts on this Biosample Hub blog include:
- Why Academic / Hospital Biobanks Should Provide Biosamples to Industry
- Ensuring Public Support For Biobank Cooperation With Industry
- The Main Actors Providing Biosamples To Industry
- Biosample Needs of Different Industry Players
- Acceptable Transactions in Biobanking
- What is Commodification?
- Why Biobank Access Policies Should Be Publicly Available
- Biospecimen Provenance: What Researchers Need To Know